Alzheimer’s disease: mysteries and new leads
These article was originally published in L'Édition N.21.
Alzheimer’s disease is a neurodegenerative pathology that affects almost one million French people today, according to the Paris Brain Institute (ICM). The disease, whose most significant risk factor is age, is the leading cause of dementia worldwide, according to the World Health Organization (WHO). Development, diagnosis and treatment: scientists at Université Paris-Saclay are studying every possible research angle concerning the disease.
Today, WHO figures show almost 36 million people with Alzheimer’s disease, a figure that could potentially triple by 2050. According to a study by Inserm and the University of Bordeaux, two million people in France will be affected by 2040, more than double the current number. “It is a disease with a very high prevalence and incidence rate,” says Yvette Akwa, researcher at the Diseases and Hormones of the Nervous System Laboratory (DHNS – Univ. Paris-Saclay, Inserm). “The main risk factor for Alzheimer’s disease is age. As we are living longer, the number of cases will increase in future years,” says the researcher. “However, age is not the only risk factor, as women are more affected (60% of French patients are women, according to Inserm) due to the drop in oestrogen during menopause. Heredity, high cholesterol and environmental agents (smoking, alcohol consumption, depression, etc.) are the other notable risk factors.”
What do we know about Alzheimer’s disease?
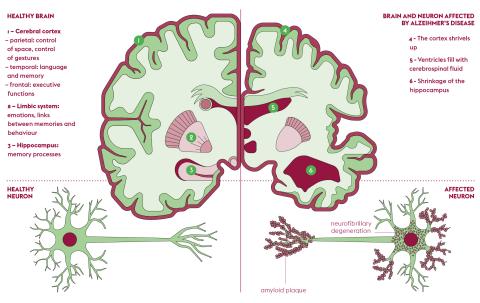
Discovered by German psychiatrist Aloïs Alzheimer in 1907, the disease that now bears his name is defined by two types of lesions affecting the brain, each caused by a specific protein: amyloid, or “senile”, plaques are due to the beta-amyloid peptide, and neurofibrillary degeneration is linked to the hyperphosphorylation of the Tau protein. These two lesions first develop in the hippocampal region of the brain, the seat of memory. The main symptom of Alzheimer’s disease - memory loss – comes from this specific location.
If the origins of the disease were to be traced, the amyloid precursor protein (APP) would be near the top of the family tree. “As implied by its name, APP is the precursor of amyloid, whose accumulation in the brain causes senile plaques. Depending on how it is cleaved by enzymes, it is at the origin of two pathways: a predominantly physiological, non-amyloid pathway, and another pathway, whose existence has not been explained, at the origin of the beta-amyloids Aβ40 et Aβ42, among others. It is this last path that is pathological, says Yvette Akwa. “Otherwise, APP has a very beneficial role, as the protein, whose gene is located on chromosome 21, promotes neuronal survival, synapse generation and memory capacity. Like genetic mutations, it is not clear why certain enzymes specifically cleave APP to produce amyloid-beta peptides.”
Aβ40 and Aβ42 peptides, derived from APP cleavage, join together progressively, first in the temporal lobe of the brain, near the hippocampus, and then spreading to the entire brain. In parallel to this increasing accumulation outside the neurons, neurofibrillary degeneration strikes inside the nerve cells, through hyperphosphorylation of the Tau protein. Normally, phosphorylation is a natural protein conformation modification mechanism through the addition of a phosphate group to one or more amino acids of the protein, thus modifying its activity. “The Tau protein promotes the polymerisation of tubulin into microtubules, in the transport of various components in the neuron. Tau has many phosphorylation sites, says Yvette Akwa. In fact, as soon as the Tau protein is hyperphosphorylated, it dissociates from the microtubules (which will then disintegrate), falls into the cytoplasm and progressively aggregates to form fibrillar neurodegeneration, leading to the death of neurons.”
This double lesion mechanism that characterises Alzheimer’s disease is very slow and generally develops over several decades before the first symptoms appear, including memory disorders (amnesia), language disorders (aphasia) and movement disorders (apraxia).
Glial cells, imaging and early identification of the pathology
The Neurodegenerative Diseases Laboratory (LMN – Univ. Paris-Saclay, CEA, CNRS) carries out the fundamental and mechanistic study of three major neurodegenerative diseases: Alzheimer’s, Parkinson’s and Huntington’s. “The singularity of the LMN lies in our transverse approach to the problem, with studies on the fundamental mechanisms, the cellular interactions and their behaviour, and the use of brain imaging techniques,” says Gilles Bonvento, Director of the laboratory. Within the LMN, there is a particular focus on glial cells, which make up about half the brain and are rarely studied in the context of neurodegenerative diseases.
As their name indicates, these diseases result in the death of neurons, and research has long focused on their operation and the mechanisms related to neurodegenerative pathologies. “We realised late in the game that in Alzheimer’s disease, for example, we were not dealing with a ‘cell-autonomous’ disease involving only neurons. Lastly, the death of the neuron is a very late phenomenon in the evolution of the disease,” says Gilles Bonvento. “The circuit failures that account for the clinical symptoms are themselves preceded by clinically silent and slowly progressing cellular processes, at least 20 years before neuronal death. We are now studying these events prior to neuronal death, incorporating new cells, such as glial cells, into the research.”
Glial cells are divided into three categories (astrocytes, microglia and oligodendrocytes) and promote communication between neurons. “Neurons are at the heart of brain activity, but evolution has made them very vulnerable. They require a lot of support cells to function properly,” says Gilles Bonvento. “Our stock of neurons at birth only decreases over the course of our life. Glial cells are not as sensitive and vulnerable to time.”
At the LMN, a dedicated team is working on the contribution of glial cells to the pathophysiology of Alzheimer’s disease. It is primarily interested in one type of glial cell in particular, namely astrocytes. One team, led by Caroline Escartin, is studying the interactions between astrocytes and neurons and the phenotypic change in these astrocytes, for example. “This opens up the possibility of identifying new biomarkers for the disease,” adds Gilles Bonvento. “It is essential to detect the disease as early as possible. It is now known that dysfunctions appear long before the first symptoms (memory, orientation or other cognitive problems). Our goal is to have biomarkers at the earliest possible stages of Alzheimer’s disease to identify and flag individuals on a trajectory that will lead to the disease.”
The LMN also benefits from its location within the Molecular Imaging Research Center (MIRCen – Univ. Paris Saclay, CEA), located at the CEA centre in Fontenay-aux-Roses and recently equipped with a particle accelerator, the cyclotron, a positron emission tomography (PET) imaging platform and magnetic resonance imaging (MRI). “The cyclotron is essential for the on-site synthesis of radioligands, which will help us to identify biomarkers of Alzheimer’s disease through PET imaging. The development of imaging tools to monitor the evolution of the pathology atraumatically is one of the LMN’s main research areas,” says Gilles Bonvento. “Today, the monitoring and diagnosis of the pathology must combine batteries of cognitive tests with other examinations. On the one hand, we are developing ligands of interest for monitoring the disease, and on the other hand new MRI approaches to objectify functional changes in the brain. This area uses several imaging modalities such as nuclear magnetic resonance spectroscopy (MRS).”
MRS is a “virtual biopsy” and provides researchers with information on the brain levels of a number of key indicators (amino acids such as glutamate, etc.). Julien Valette, Assistant Director of the LMN, and his team have also developed new spectroscopic methods to quantify certain morphological characteristics of neurons and astrocytes. But this is not a single solution. “With Alzheimer’s disease, the difficulty is being selective and sensitive to early events. Lastly, we are seeking to develop a set of relevant arguments to characterise the disease as early as possible. It is likely to be difficult to tell whether a patient has the disease in a single NMR spectroscopy examination. On the other hand, NMR provides additional arguments, which then converge with clinical tests and PET imaging, to make a definitive diagnosis and especially to monitor the effectiveness of therapies.”
Steroids sulphates as treatment?
At the DHNS, Yvette Akwa is interested in pregnenolone, the main precursor of neurosteroids, which are steroids synthesised in the nervous system from cholesterol. “I first became interested in the biosynthesis and metabolism of neurosteroids in rat brains,” says the researcher. Pregnenolone is converted to pregnenolone sulfate in the body. In addition, it has been established that this sulfated molecule stimulates the glutamatergic N-methyl-D-aspartate (NMDA) receptors present at the synapses, the interface between two neurons ensuring the transmission of information between them. NMDA receptors are essential to memory and to the ability of the nervous system to make and break new connections between neurons, which is known as synaptic plasticity.
Furthermore, positive effects of pregnenolone sulfate on memory have been observed. “I have shown that pregnenolone sulfate was promnesiant, that it promoted spatial memory performance, in young animals (mice and rats), and antiamnesiant in animals aged between 12 and 21 months. It was from these discoveries that I became interested in Alzheimer’s disease, as pregnenolone sulfate was able to stimulate memory and reverse memory loss in animals not affected by the disease,” continues Yvette Akwa. According to her studies, the level of pregnenolone sulfate decreases with age, and even more rapidly when patients are affected by Alzheimer’s disease. “We quantified neurosteroid levels in samples from different brain regions in elderly patients with and without the disease. These levels have been seen to decrease significantly in the brains of patients with the disease, compared to those without it. But the most important factor lies elsewhere, as the decrease in the amount of pregnenolone sulfate in the brain correlates with high levels of amyloid deposits. Through further studies, we have shown that this neurosteroid has a protective role for the neuron and corrects the symptoms caused by amyloid-beta peptide. Recently, we have also observed a decrease in hyperphosphorylated Tau proteins,” says Yvette Akwa. The researcher has two goals, namely to eliminate the aggregates linked to the disruption of the Tau protein or beta-amyloids, and, just as importantly, to decrease or even prevent the hyperphosphorylation of Tau. Without this abnormal phosphorylation, the protein does not deposit in the cytoplasm of the cell and does not form aggregates. “I am hopeful that compounds like pregnenolone sulfate can both decrease amyloid neurotoxicity and Tau neurotoxicity and, most importantly, restore memory. We would then be fighting a real combination of the three essential aspects of Alzheimer’s disease,” explains Yvette Akwa.
Promoting interactions between transthyretin and beta-amyloid peptide
In the Biomolecules laboratory: design, isolation, synthesis (BioCIS – Univ. Paris-Saclay, CNRS, CY Cergy Paris Univ.), Nicolo Tonali is particularly interested in the aggregation of amyloid-beta peptide Aβ42 in the brain. The aim of his research is to avoid the formation of these fibrillar species and facilitate the physiological degradation of the peptide. “In amyloid plaques, we find different proteins such as transthyretin (TTR), whose main role in the blood is to transport hormones. We found that there was a myriad of positive interactions between TTR and Aβ42, such as the reduction of aggregates and their toxicity, and the degradation and dissociation of aggregates already formed,” Nicolo Tonali explains.
Since those initial findings in 2021, he and many of his colleagues have developed a strategy to bring TTR and Aβ42 together. Based on mediated proteolysis, the PROTAC (Proteolysis-targeting chimeric) strategy involved forcing the clustering of two proteins – in this case, Aβ42 and TTR – with the goal of maximising their interactions and promoting the removal of the amyloid peptide. “We observed good interaction between TTR and a portion of Aβ42 mediated by copper, a metal, incidentally, that is highly present in the brains of Alzheimer’s patients. We want to build a molecule with a peptide linker that is able to both fetch TTR and place it in contact with Aβ42, so that the physiological role of the former protein acts on the latter. The final grouping is then evacuated,” says the researcher.
Alzheimer’s disease remains an extensive and exciting research topic, particularly due to the many mysteries that still surround it. But thanks to new tools, it is becoming possible to better understand it, and researchers hope to detect it as early as possible and develop therapeutic solutions.
Publications:
- Akwa Y., Steroids and Alzheimer’s Disease: Changes Associated with Pathology and Therapeutic Potential, Int. J. Mol. Sci. 2020.
- Bonvento G., Glycolysis-derived L-serine in hippocampal astrocytes rescues synaptic plasticity and memory loss in a mouse model of Alzheimer’s disease. Alzheimer’s Dement., 2022.
- Tonali N., et al., Application of PROTAC strategy to TTR-Aβ protein-protein interaction for the development of Alzheimer’s disease drugs. Neural regeneration research, 2021.