Cutting-edge stem cell research
(Article from l'Edition n.15 - february 2021)
A great deal of research is devoted to exploring the regenerative and therapeutic potential of embryonic and induced pluripotent stem cells and their suitability for new disease study models. At the same time, adult somatic stem cells can treat severe burns and haematopoietic and corneal defects.
Embryonic stem cells (ESCs), identified in 1981 in mice and then in humans in 1998, have raised many hopes of finding treatments. This is for good reason. They are taken from an embryo at an early stage in its development and so they show promising properties. Potentially capable of multiplying ad infinitum, they are also theoretically able to reproduce all of an individual’s cell types.
In 2006, the first account of Induced Pluripotent Stem Cells (IPSCs) generated even more excitement. Produced in the laboratory from an individual’s mature somatic cells, these cells are genetically reprogrammed to return to an embryonic stem cell state. This technique is free from some of the ethical concerns linked to the manipulation of embryos.
Ensuring skin renewal
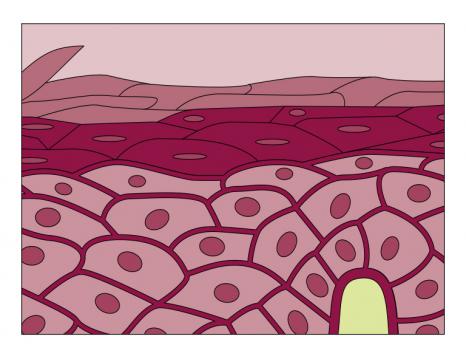
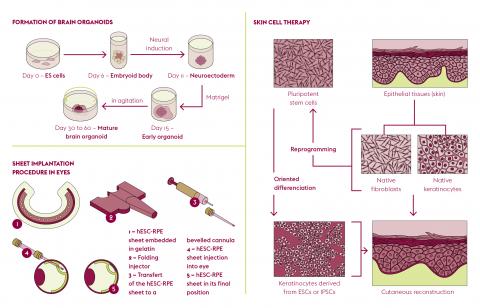
A field of research focuses on adult somatic stem cells, which are naturally present in tissues and organs. At the “Laboratoire de génomique et radiobiologie de la kératinopoïèse” (LGRK) (Genomics and Radiobiology of Keratinopoiesis Laboratory) at the “Institut de biologie François Jacob” (Université Paris- Saclay, CEA), Michèle Martin and Nicolas Fortunel study stem cells in the adult human epidermis. These ensure the homeostasis of skin tissue throughout an individual’s life. Consisting mainly of keratinocytes, the epidermis acts as the body’s first line of defence but still holds many secrets. The precise location of its precursor cells is not yet clearly understood. “Clinical and biological data show that reservoirs of keratinocytes with very high regenerative potential exist in the basal layer of the interfollicular epidermis,” explains Nicolas Fortunel. “To date, these cells remain partially characterized as their phenotype has not yet been fully established.”
To isolate the highly enriched subpopulations, the LGRK team is developing a range of markers. “We capture a situation at a given moment, which varies according to the life of the epidermis and the stimuli it has received.” “This dynamic and transient stem state varies according to the microenvironment and is distributed according to equilibrium effects,” adds Michèle Martin. The degree of quiescence (dormancy) of the cells is one of the best markers. “An immature basal cell which is very quiescent is likely to be in a stem state.” Under the effect of a stimulus – an injury, for example – this cell begins to divide, producing daughter cells which multiply. Some of them develop into mature keratinocytes, while others produce stem cells again.
A new physiopathological phenomenon
The team recently studied the impact of low-dose irradiation (comparable to that received during a CT scan) on the quiescence of epithelial stem cells. “These dormant cells were placed in the context of the active metabolism of epidermis regeneration, which woke them up and caused them to divide,” recounts Nicolas Fortunel. “They then showed an increased sensitivity to genotoxic stress which, in normal tissue homeostasis, would have no detrimental effect.”
If, on a macroscopic scale, these stem cells remain capable of regenerating a three-dimensional epidermis and correct differentiation, disorder in the stacking of keratinocytes appears in places on a microscopic scale. “These dysplastic areas show a transition from a normal epithelial state to a mesenchymal state. The keratinocytes assume the characteristics of fibroblasts. This phenotypic change corresponds to the early stages of malignant transformation.”
Better defined growing media
In theory, epithelial stem cells can be kept for years in a culture. “It has been calculated that we could graft the whole of humanity from a single cell cultivated under the best conditions!” points out Michèle Martin. “However, the greatest stumbling block – not damaging this potential as the growth increases in a culture – still remains.”
Until recently, epithelial stem cell culture was carried out in media containing derivatives from animals, such as serum or growth drivers which were very effective but not very well defined. The keratinocytes used clinically fall into this category. However, regulatory authorities are now imposing new research constraints on laboratories, leading to well-defined environments which are free of animal proteins. “There is an acute need to revisit the conditions for growing stem cells in culture in order to pinpoint the factors which are absolutely necessary to conserve their potential.” “This opens up a new field of research combining molecular biology, genomics and epigenomics,” enthuses Nicolas Fortunel.
The KLF4 pathway
This is also a new gateway that the team, in collaboration with colleagues from the CEA at Fontenay-aux-Roses and the “Institut des cellules souches pour le traitement et l’étude des maladies monogéniques” (Stem Cell Institute for the Treatment and Study of Monogenic Diseases) (I-Stem – Université Paris-Saclay, INSERM, Université d’Évry), has just opened. The researchers have shown the role of the transcription factor KLF4 (Kruppel-like factor 4) in the maintenance of human keratinocytes. By reducing its level of expression using RNA interference or by pharmacological repression, they have encouraged the immaturity of stem and keratinocyte progenitor cells and their self-renewal when growing in culture.
The team, which enjoys a long-standing collaboration with the Percy hospital which specialises in serious burn victims, now plans to explore the KLF4 pathway in preclinical models in order to succeed in preserving stem cells qualitatively and quantitatively during the bioengineering phases of grafting and to improve their regenerative potential. It is also focusing on new classes of epigenome effectors, such as non-coding RNAs. This is a still unexplored field of research in the case of stem cells. “The coding genome is not enough to subtly control this stem state. We are therefore going to look at these RNAs to try to explain the plasticity of epidermal stem cells and the balance of this tissue.”
A retina patch for cell therapy
At I-Stem, Christelle Monville and her team are using cell therapy to treat age-related macular degeneration and certain forms of retinitis pigmentosa, which are characterised by a degeneration of the photoreceptor cells in the retina. Although the retinal pigment epithelium (RPE) is not directly involved in the conversion of light signals received by the eye into electrical impulses transmitted to the brain by photoreceptors, it is nonetheless essential for vision. This layer adjacent to the photoreceptors secretes proteins which are essential for their proper function, removes waste and absorbs excess light. It is also affected in most retinal diseases.
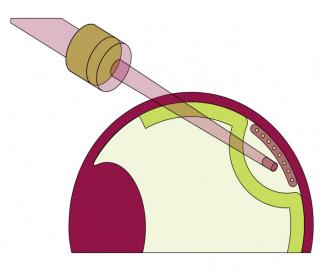
The team, together with the “Institut de la vision” (Sorbonne Université, INSERM, CNRS) has developed a patch of RPE cells derived from human pluripotent stem cells. “RPE cells are easier to obtain in terms of quantity, functionality and purity from pluripotent stem cells than the highly-specific and fragile photoreceptor cells. Not to mention that the maturation of photoreceptor cells continues after birth and that this process is difficult to reproduce in a culture,” points out Christelle Monville. The idea is then to implant this patch, which is 3 mm wide by 5 mm long and in the shape of a SIM card, in the macula in the eye very early in the development of the disease in order to preserve central vision by slowing down or stopping the death of the photoreceptors.
In contrast to procedures involving cells in suspension, here the RPE cells are placed on a human amniotic membrane obtained from Saint-Louis Hospital’s tissue bank. “In animals, this has been shown to increase the chances of the integration, survival and functionality of RPE cells, which must be structured to function properly.”
A human clinical trial
After conclusive trials in rodents and primates, the team launched a phase 1-2 clinical trial in humans in autumn 2019, financed by AFM-Telethon and targeting 5% of retinitis pigmentosa. The trial involves a total of twelve patients, divided into two cohorts. The patch is produced by the French blood establishment (EFS) ABG in Nantes for one month and the Quinze-Vingts National Ophthalmology Hospital in Paris carries out the implantation in the patients’ eyes. For this trial, the team also called on the expertise of the Clermont-Ferrandbased company, Medical Device Engineering, which designed a three-cap plastic mould system to coat the patch with gelatine for better handling.
At the end of the transplant, patients are put on immunosuppressive drugs for one year and undergo very close medical monitoring. Fourteen medical visits are scheduled during the first year, then one every six months for the next four years, together with blood tests, visual tests and imaging. “The first two patients, who had very poor eyesight, had the transplant in September and October 2019. The aim was to test the safety and tolerance level of the patch. To date, they are doing very well. They have tolerated their immunosuppressive treatment well and the graft has not been rejected. They even described a small improvement in their perception of light.” Because of the Covid-19 health crisis and the first lockdown in the spring, transplantation for the ten patients in the second cohort was delayed and the new implantations started in October 2020. They will continue until the end of 2021 or the beginning of 2022. “These patients are less affected as their illness is more recent. We hope that they will still have some functional photoreceptors present and that we will be able to save them and see some functional benefit from the graft.”
Today, Christelle Monville’s team are looking at the other 95% of retinitis pigmentosa, which involves mutations in photoreceptor genes. ‘”We are embarking on a collaboration with the “Centre de nanosciences et de nanotechnologies” (Centre for nanoscience and nanotechnology, C2N – Université Paris-Saclay, CNRS) to work on more complex patch structures with photoreceptors integrated into a synthetic membrane under development at C2N, which will be able to reproduce the particular orientation of these cells in the retina. We are also going to have to alter the scale so we can produce more patches for the market and work on freezing them to keep them longer.”
The complexity of neurodegenerative diseases
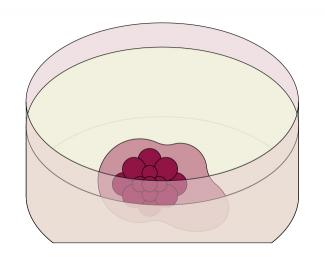
At the “Service d’étude des prions et des infections atypiques (Department for the study of prions and atypical infections, SEPIA – Université Paris-Saclay, CEA), Jean-Philippe Deslys and Frank Yates from Sup’Biotech are developing new in vitro and three-dimensional models of neurodegenerative diseases. Obtained from induced pluripotent stem cells resulting from the reprogramming of skin fibroblasts from an individual (affected or not by a disease), these models exploit the extraordinary self-assembly potential of pluripotent cells. After one week of culture, these cells develop into neural cells and form a small ball called an embryoid body. Then, in the presence of a hydrogel which mimics the cellular environment, in two months the embryoid body becomes a sphere 2-3 mm in diameter and a mature brain organoid, whose development automatically stops at a foetal stage. Without possessing all cell types (brain organoids contain only neurons and glial cells) certain regions of this “mini-brain” resemble a developing human brain.
“When we look at the often progressive evolution of diseases affecting the human brain, animal models fail to show all the physiopathological signs observed in humans,” points out Frank Yates. Other ways of analysing the underlying molecular mechanisms must be sought beyond the already well-characterised two-dimensional models. This is where the brain organoids developed by the SEPIA and Sup’Biotech team come into play, particularly for Alzheimer’s disease.
This disease is characterised by slow neuronal degeneration and the formation of lesions in the brain, accompanied by clumping of the proteins beta-amyloid and Tau. While the peptide form Aβ42 of the protein β-amyloid clumps as plaques between neurons, a hyperphosphorylated form of the protein Tau clumps inside neurons in the form of neurofibrillary tangles. “However, it is not known whether it is these clumps which cause neuronal degeneration, or whether it is the neuronal degeneration that is involved in the formation of the clumps,” points out Frank Yates. Basically, it is a chicken and egg problem.
Developing new tools
“Using brain organoids and genetic engineering techniques, the laboratory is equipped with tools capable of summarising certain physiopathological signs of Alzheimer’s disease in order to better understand its mechanisms,” says Frank Yates. To model the disease, the researchers, in collaboration with the “Laboratoire Croissance, réparation et régénération tissulaires” (Tissue Growth, Repair and Regeneration Laboratory, CRRET – UPEC, CNRS), are generating a mutated form (p301S) of the Tau gene from a patient suffering from frontotemporal dementia. The condition, similar to Alzheimer’s disease, is characterised by Tau phosphorylation. By forcing the expression of p301S-Tau in the brain organoids, researchers are inducing this disease-causing characteristic which is so important in the development of neurodegenerative diseases.
“However, to try to understand what happens inside an organoid is to be confronted with the third dimension and its technical difficulties,” points out Jean-Philippe Deslys. To bypass these, the laboratory has an ultramicroscope which allows the organoids to be observed once they have been made transparent using a chemical process. “We are living in a time of technological revolution, and the combination of these technologies is phenomenally powerful,” adds Jean-Philippe Deslys.
In collaboration with François Treussart’s team from the “Laboratoire Lumière, matière et interfaces” (Light, material and interfaces laboratory, LUMIN – Université Paris-Saclay, CNRS, ENS Paris-Saclay, CentraleSupélec) and Marco-Antonio Mendoza-Parra’s team from the “Laboratoire Génomique métabolique” (Metabolic genomics laboratory – Université Paris-Saclay, Université d’Évry, CNRS, CEA), Jean-Philippe Deslys’ team is currently developing a cross-disciplinary research project focused on the study of the early stages of Alzheimer’s disease using mini-brains. Funded by the French Foundation for Medical Research, the project involves carrying out a 3D mapping of the expression of the genes involved, producing nanocrystals for recording physiological phenomena and monitoring the electrophysiological activity of the minibrains in order to gain a better understanding of what underlies these physiopathological clumps. “We hope to bring our initially embryonic model closer to an ageing brain by forcing the mechanisms which lead to the clumping of abnormal beta-amyloid and Tau proteins,” concludes Frank Yates.
Publications :
- Fortunel NO, Martin MT. When the Search for Stemness Genes Meets the Skin Substitute Bioengineering Field: KLF4 Transcription Factor under the Light. Cells, 9 (10), (2020).
- Karim Ben M'Barek, et al. Clinical-grade production and safe delivery of human ESC derived RPE sheets in primates and rodents, Biomaterials, 230, (2020).
- Nassor Ferid, et al. Long Term Gene Expression in Human Induced Pluripotent Stem Cells and Cerebral Organoids to Model a Neurodegenerative Disease. Frontiers in Cellular Neuroscience, 14, (2020).